Rank the Structures Shown From Most to Least Stable.
Solution for Rank the structures shown from most to least stable. Rank them according to decreasing atomic number.

How To Choose The More Stable Resonance Structure Chemistry Steps
Meanwhile the least stable structures are known as the minor contributors.
. - III IV A. Previous question Next question. What is the expected major products of HCl.
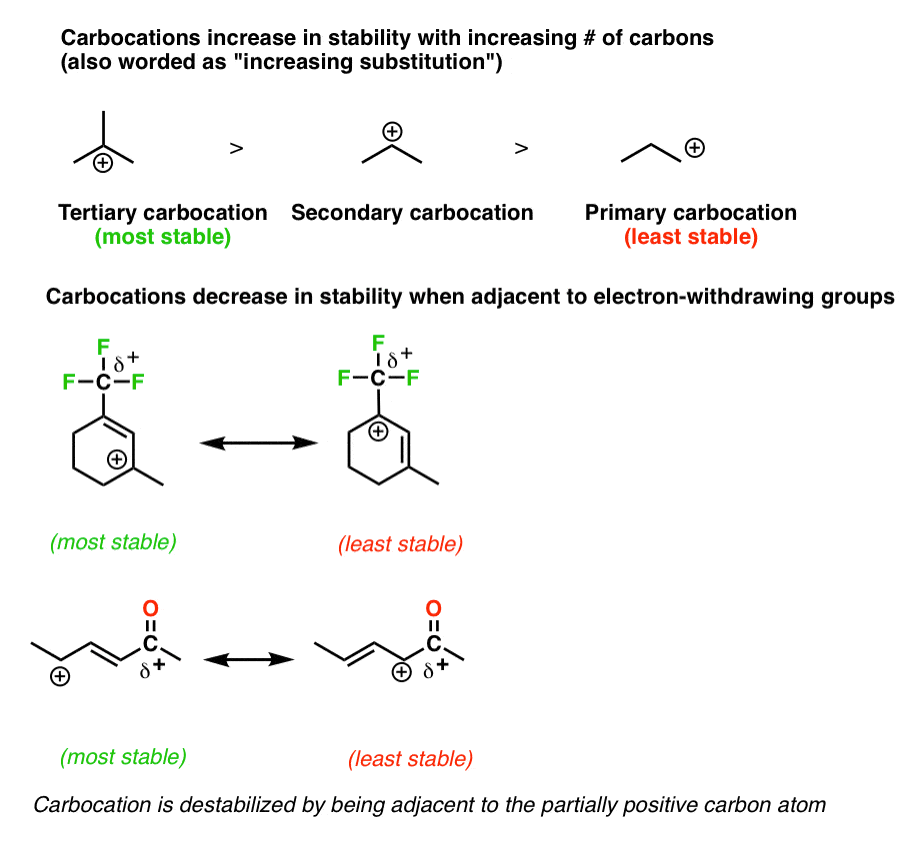
But what happens if a carbocation is allylic ie. Lone pairs are not explicitly shown think about the number of lone pairs implied by the bond line structures. That are possible the more stable the carbocation.
100 5 ratings Transcribed image text. Two molecules reacting to form a single molecule. The least stable highest energy conformation will have an eclipsed conformation.
14 Inductive Effects section 21. Which of the structures shown depicts the most stable carbocation intermediate formed in the hydrohalogenation reaction shown. 15 points Write clear structures that show the following.
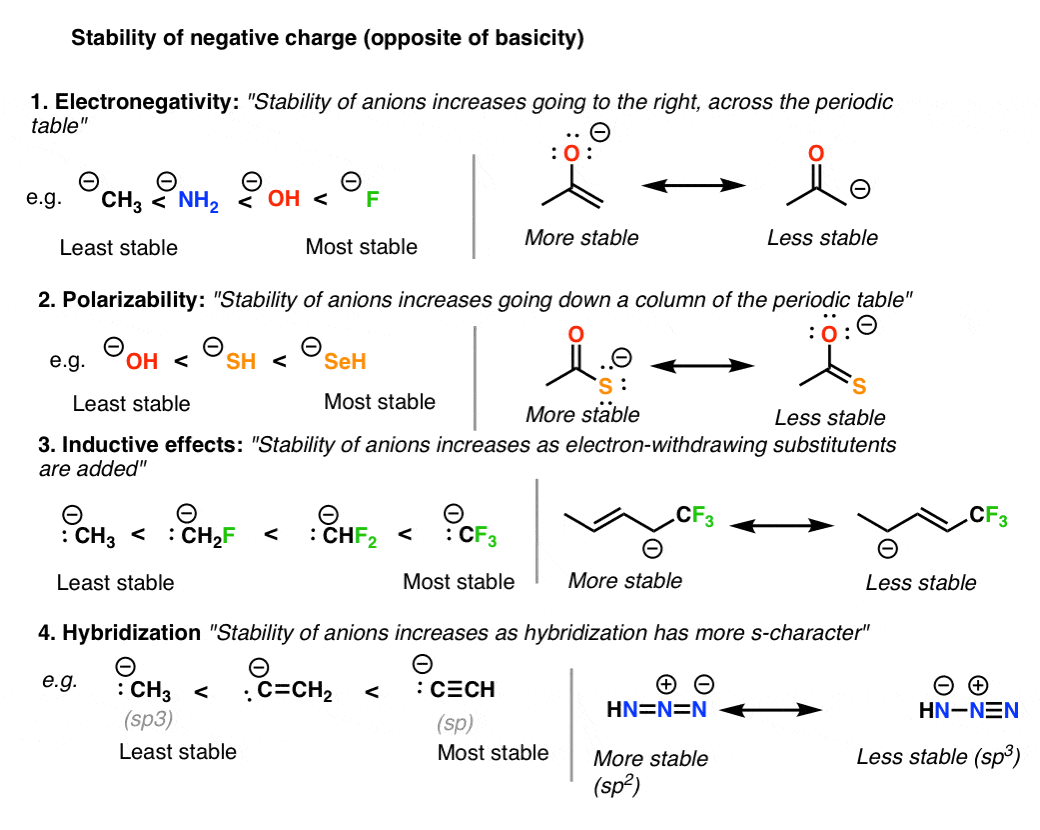
A handy way of determining the substitution alternatives is to use the Haworth. What is important as well is that not all the resonance structures are equally stableIn fact the most stable resonance form is the resonance hybrid since it delocalizes the electron density over a. If two structures are equal they will contribute equally Resonance Stability Rules.
Remember resonance structures have the same placement of atoms meaning that they represent the same compound and only the arrangement of electrons is different. The first step in drawing the most stable conformation of cyclohexane is to determine based on whether the substituents are cis or trans to one another and based on where theyre located on the ring what the choices of axial and equatorial positions are for the substituents. C a compound with five carbons all of which are sp2 hybridized.
Rank the structures shown from most to least stable. Answer to Solved Rank the structures shown from most to least stable. Experts are tested by Chegg as specialists in their subject area.
Crystal violet is the common name for the chloride salt of the carbocation whose structure is shown below. Rank the following base pairs according to their stability. C a b.
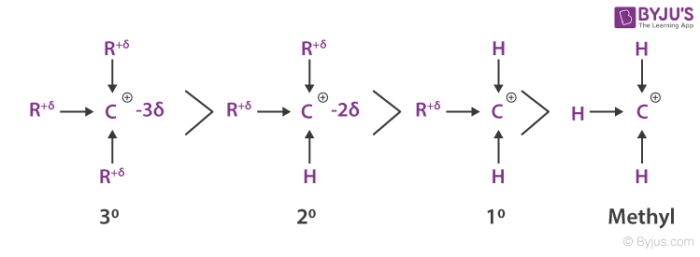
III IV. Weve sorted carbocations in order of decreasing stability. This is the best answer based on feedback and ratings.
1 II OT Il IN O III II 1 O II III 1 O II 1 III O I Ill 11. However there are some unusual examples of very stable carbocations that take the form of organic salts. The stability order of given alkenes are Option E- IIIIII Reason.
05 pts 2 Which position would react at the fastest rate with a bromine radical. Adjacent to a double bond. For the most part carbocations are very high-energy transient intermediate species in organic reactions.
Chemistry questions and answers. A the best Lewis structure for HCNO bonded in that order b the Lewis acid-base reaction between BF3 and CH3OH. View the full answer.
What is the alternate chair conformation of the following compound. Rank the structures shown from most to least stable. Question 3 Rank the structures shown from most to least stable.
Rank the following carbocations a -c in order of increasing stability from least stable to most stable. I II III IV B. Shifting of electrons in a s-bond in response to the electronegativity of a nearby atom or group.
II III IV 1 C. II III OI 111 O III 11 1 O 11 III 1 O 111. To find out which resonance structure is the most stable there are five main rules to follow.
Solve any question of Organic Chemistry - Some Basic Principles and Techniques with-. D all possible isomers with the name dimethylcyclopropane. To rank items as equivalent overlap them.
For each of the following draw the best most stable and worst least stable Newman projection relative to the bond indicated in each question. Which of the structures shown depicts the most stable carbocation intermediate formed in the hydrohalogenation reaction shown. IV 2Rank the conformers of 1 2 4-trimethylcyclohexane in order of decreasing stability putting the most stable first.
The most stable conformations will be staggered conformations with the largest groups ANTI to each other. Hence the correct option is A. Tertiary is on top since its the most stable due to its R-groups and methyl is on bottom because it has no R-groups.
We review their content and use your feedback to keep the quality high. Show transcribed image text. Rank the resonance structures below from most to least important.
Rank the following aromatics in order of decreasing reactivity toward electrophilic aromatic substitution Most reactive least reactive. Africas Top 5 most politically-stable countries. 05 pts A 1 B 2 C 3 D 4 CH2-CH2-CH3 3 Predict the major.
Rank the structures shown crom most to least stable. Rank the following carbocations in order of decreasing stability. 1Il IV 11 III IV III IV 11 V DIV.
According to saytzeff Rule. Consider all valid resonance structures of the molecule shown below. Rank the structures shown from most to least stable.
Lots of possibilities such as. We review their content and use your feedback to keep the quality high. Rank from most to least stable.
The most stable structures are known as the major contributors. Populous South Africa ranked number six on ACBRs country ranking for political stability The underlying factor that has created a foundation of stability on which these nations have been built is a serious will by the citizenry and their elected representatives to sustain democratic. Rank the structures shown from most to least stable.
3 2 4 1. ОН H 도 도 - II III A B I D E. 1 Rank the following radicals in order of decreasing stability I most stable.
Among the given resonance structures the least stable resonance structure is given by option A as there is minimum charge separation between the like charges which increase and decrease the stability. With the most Hs the least substitutent carbon and the X will add. Least stable most reactive lone pair lone pair able to delocalize.
Rank the following structures from most to least important Lone pairs are not explicitly shown 3 2 4 1.
Carbocation Stability Definition Order Of Stability Reactivity
Resonance Structures 4 Rules On How To Evaluate Them With Practice

How To Choose The More Stable Resonance Structure Chemistry Steps

How To Choose The More Stable Resonance Structure Chemistry Steps

Batsto Village Is A New Jersey Historic Site And Is Listed On The New Jersey And National Registers Of Historic Places There Are House Interior House Interior

How To Choose The More Stable Resonance Structure Chemistry Steps
15 3 Pi Molecular Orbitals Of Benzene Chemistry Libretexts
Resonance Structures 4 Rules On How To Evaluate Them With Practice
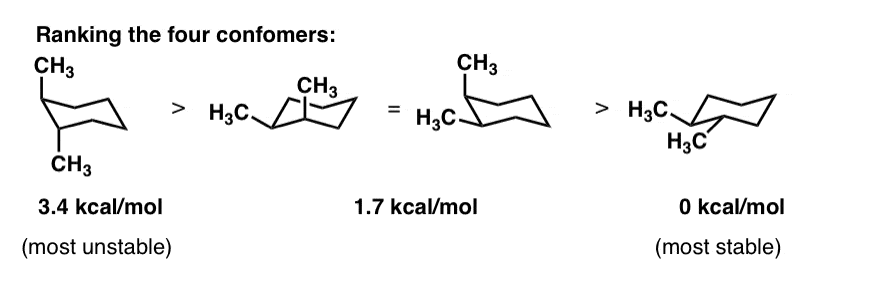
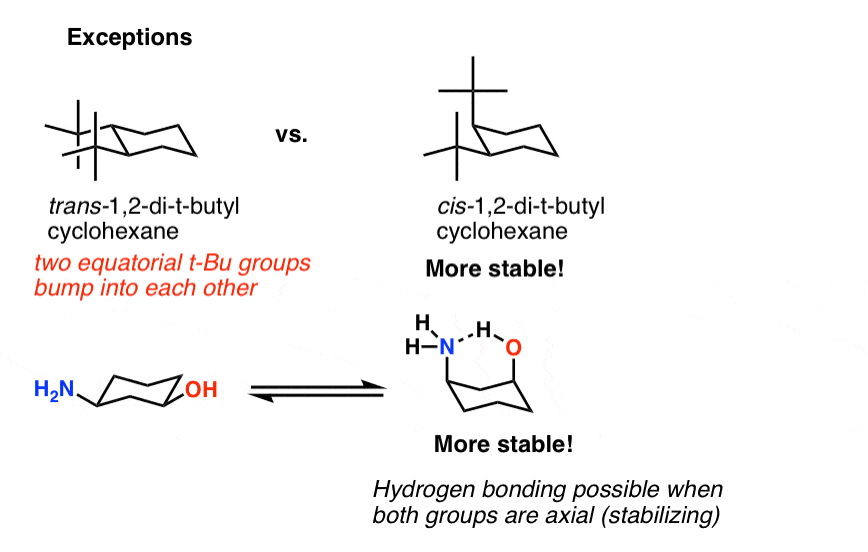
Cyclohexane Chair Conformation Stability Which One Is Lower Energy

3 Factors That Stabilize Carbocations Master Organic Chemistry Organic Chemistry Organic Chemistry Reactions Study Chemistry

Resonance Structures 4 Rules On How To Evaluate Them With Practice Organic Chemistry Chemistry Help Chemistry Worksheets

How To Choose The More Stable Resonance Structure Chemistry Steps

Pin On Adto Scaffolding Planks For Sale
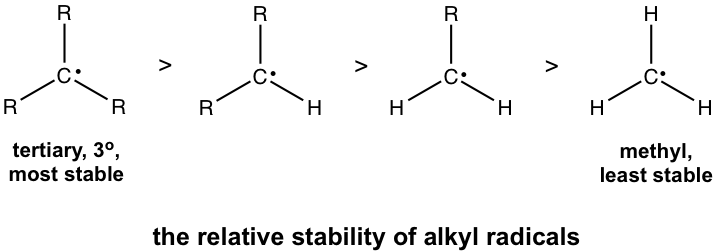
9 3 Stability Of Alkyl Radicals Organic Chemistry I

Cyclohexane Chair Conformation Stability Which One Is Lower Energy

Cyclohexane Chair Conformation Stability Which One Is Lower Energy

How To Choose The More Stable Resonance Structure Chemistry Steps

Comments
Post a Comment